Our Scientific Advisors
Our Scientific Advisory Board guides us through the research and development process to cure Batten disease. Our initial primary focus is on Batten CLN6, but our goal is to expand to other Batten disease variants.

David A. Pearce, Ph.D
President of Sanford Research, Sanford Health Director at Sanford Children's Health Research Center, and Sanford Research Professor in the Department of Pediatrics at Sanford School of Medicine of the University of South Dakota.

Clive Svendsen, Ph.D
Director of the Board of Governors Regenerative Medicine Institute at Cedars-Sinai Medical Center, Professor of Medicine and Biomedical Sciences holding the Kerry and Simone Vickar Family Foundation Distinguished Chair in Regenerative Medicine, and Board Member at Emulate Incorporated.

Jill Weimer, PH.D
Dr. Jill Weimer, a developmental neuroscientist, serves as Chief Science Officer at Amicus Therapeutics and Senior Director of Therapeutic Development at Sanford Research. Her lab initiated the first gene therapy trials for CLN3 and CLN6 Batten disease. She earned her B.S. and Ph.D. in neuroscience from the University of Rochester and completed postdoctoral training at UNC–Chapel Hill.
Our Research Areas
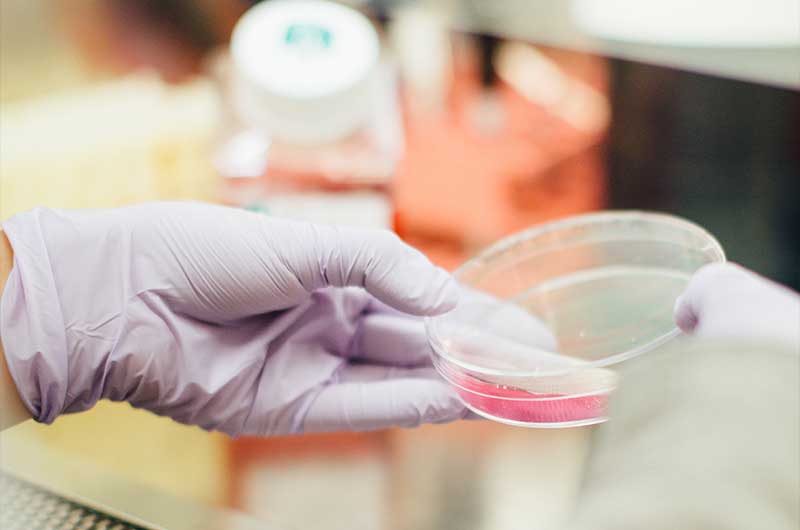
CELLULAR THERAPY
We are optimistic about the potential for research into restorative therapies involving stem cell transplantation. These therapeutic cells are customized and then applied to damaged areas of the brain. While in an earlier stage of development, the potential for brain regeneration is no longer in the realm of fiction.
Funding research that could result in the rejuvenation of lost brain cells is a priority for the foundation, but the costs are massive. Progress on this front would have significant impact not only for Batten disease patients, but individuals suffering from neurodegenerative disorders of all kinds. Help us get there by supporting the foundation’s research goals financially.

SMALL MOLECULE THERAPY
Our research team has identified a targeted approach to validate novel therapeutics for Batten disease using already FDA approved drugs, such as GRAS nutraceuticals and designer molecules that can potentially correct the mutated gene. This validation method involves patients’ own cells in culture to assess the effect of these compounds on restoring the function of the abnormal protein.
As these agents are mostly safe for human use, there is high potential for lead compounds to move quickly into clinical trial, making them an exciting avenue as we seek a cure for Batten disease. With your support, we can get these therapies to clinical trials even faster.

GENE THERAPY
We are working with the world’s leading researchers studying gene transfer approaches to pediatric orphan diseases, and thanks to truly groundbreaking research supported by the foundation, we’ve made some exciting advances.
One of the biggest obstacles for treating any brain disease is the inability of nearly all drugs and treatments to cross through the blood brain barrier, which prevents the entry of external compounds into the brain. Because of this barrier, potentially lifesaving treatments aren’t able to reach their intended target. But now, thanks to research supported by the foundation, scientists have discovered an ingenious way to overcome this barrier.
This method relies on nature itself and on the behavior of a particular virus called Adenovirus (AAV). Through millions of years of evolution, AAV has figured out ways of infecting cells, including the brain, and now scientists have devised methods to harness this power while removing its infectivity. The replacement gene is delivered to the brain by inserting it into the AAV virus, allowing nature itself to deliver this potentially lifesaving technology.
The malfunctioning of the mutated CLN6 gene is what causes Batten disease, and the replacement gene appears to help restore the functionality of the mutated gene. This allows cells to purge built-up wastes and restore balance in the brain. A first of its kind clinical trial has begun at Nationwide Children’s Hospital to evaluate this investigative treatment, providing hope for children with Batten disease all around the world.
With your help, the foundation is committed to providing this potentially lifesaving opportunity to all eligible children.
Published Scientific Research on Batten CLN6

Gene Therapy Corrects Brain and Behavioral Pathologies in CLN6-Batten Disease
CLN6-Batten disease, a form of neuronal ceroid lipofuscinosis is a rare lysosomal storage disorder presenting with gradual declines in motor, visual, and cognitive abilities and early death by 12–15 years of age. We developed a self-complementary adeno-associated virus serotype 9 (scAAV9) vector expressing the human CLN6 gene under the control of a chicken β-actin (CB) hybrid promoter. Intrathecal delivery of scAAV9.CB.hCLN6 into the cerebrospinal fluid (CSF) of the lumbar spinal cord of 4-year-old non-human primates was safe, well tolerated, and led to efficient targeting throughout the brain and spinal cord. A single intracerebroventricular (i.c.v.) injection at post-natal day 1 in Cln6 mutant mice...

Single-dose AAV9-CLN6 gene transfer stabilizes motor and language function
Variant late infantile neuronal ceroid lipofuscinosis 6 (vLINCL6), or CLN6 Batten disease, is a fatal neurodegenerative disorder for which there is no treatment1,2
Affected children experience language delay, motor regression, intractable epilepsy, and vision loss, leading to early death in childhood1,2
The objectives of the study are to evaluate safety and efficacy of a single intrathecal injection of AT-GTX-501, a non-replicating, recombinant, self-complementary AAV9 vector containing the human CLN6gene, into the lumbar spinal cord for the treatment of CLN6 Batten disease

Intracranial delivery of AAV9 gene therapy partially prevents retinal degeneration
Batten disease is a family of rare, fatal, neuropediatric diseases presenting with memory/learning decline, blindness, and loss of motor function. Recently, we reported the use of an AAV9-mediated gene therapy that prevents disease progression in a mouse model of CLN6-Batten disease (Cln6nclf), restoring lifespans in treated animals. Despite the success of our viral-mediated gene therapy, the dosing strategy was optimized for delivery to the brain parenchyma and may limit the therapeutic potential to other disease-relevant tissues, such as the eye. Here, we examine whether cerebrospinal fluid (CSF) delivery of scAAV9.CB.CLN6 is sufficient to ameliorate visual deficits in Cln6nclf mice. We show that intracerebroventricular (i.c.v.) delivery of scAAV9.CB.CLN6
Clinical Trial

Principal Investigator: Dr. Olivia Kim-McMannus
Site: Rady’s Children’s Hospital at UCSD
For information on inclusion criteria and more, please visit clinicaltrials.gov
For speaking inquiries, questions, and press / media please contact: [email protected]
Olivia Kim-McManus, MD, is a physician scientist and principal investigator funded by the National Institutes of Health/National Center for Advancing Translational Science and California Institute for Regenerative Medicine. Her research focuses on novel cutting edge genetic therapies including the development and delivery of antisense oligonucleotides for rare epilepsies and neurodegenerative disorders, intracranial EEG monitoring, and epilepsy surgery.
Dr. Olivia Kim-McManus is a triple Board-certified child neurologist with special qualification in child neurology, epilepsy, and clinical neurophysiology specializing in the care of infants, children, and adolescents with neurological disorders, particularly monogenetic etiologies associated with developmental delay and epileptic encephalopathy. She has extensive expertise in discovery and clinical trial for novel targeted cell and gene based therapies, clinical neurophysiology and epilepsy surgery, including invasive intracranial EEG monitoring and intraoperative electrocorticography.
She has unique expertise as PI for personalized n=1 genetic therapy, allele selective ASO discovery and delivery, FDA IND holder, and intrathecal ASO delivery and intraventricular targeted therapies for pediatric genetic disorders, with related publications in Nature Biotechnology and Nature Communications regarding diagnostic odysseys and n=1 genetic therapy trials.
Dr. Kim-McManus serves as an Associate Professor in the UCSD Department of Neurosciences and American College of Graduate Medical Education pediatric epilepsy fellowship director. She established and directs the Rady Precision Therapeutics Neuro-Interventional Program for rare disease therapies including children with Batten Disease, a rapidly progressive neurodegenerative disorder, for whom she delivers disease modifying intraventricular targeted therapy.
Dr. Kim-McManus received her undergraduate degree in Neurosciences at Columbia University in New York City, medical degree at The George Washington University School of Medicine Children’s National Medical Center in Washington DC, residencies in pediatrics and neurology at USC Children’s Hospital Los Angeles, and fellowships in epilepsy and clinical neurophysiology at UC Irvine.
She serves on numerous Advisory Boards and research committees/initiatives including Illumina, CureNDD, Combined Brain, KCNT1 Foundation, CureKCNH1 Foundation, N=1 Collaborative SAB, Praxis Precision Medicines, Stoke Therapeutics, Biomarin, UCB Pharmaceuticals, Care & Cure Institute, American Epilepsy Society Translational Research Committee, Child Neurology Society Research Committee, Pediatric Epilepsy Research Consortium Research Committee, Altman Clinical and Translational Research Institute.
She recently presented her n=1 ASO research at numerous national meetings including the 150th Anniversary American Neurological Association Plenary on precision therapies, ASO and multiomics in Baltimore MD (September 2025) and the Child Neurology Society Presidential Symposium on targeted genetic therapies in Charlotte NC (October 2025).
FAQ
WHAT IS BATTEN DISEASE?
Batten disease is a rare, genetically inherited disorder that belongs to a group of progressive degenerative neurometabolic disorders known as the neuronal ceroid lipofuscinoses (NCLs).[1]
NCLs are characterized by genetic mutations which disrupt cells’ ability to dispose of wastes, resulting in the abnormal accumulation of certain proteins and lipids (fats) within the nerve cells of the brain and other tissues of the body. This results in progressive neurological impairment including developmental regression, seizures, blindness, behavior changes, and dementia.
There are many forms of NCL. Mutations in at least eight different genes are known to cause Batten disease.[2]
Charlotte and Gwenyth have been diagnosed with Late Infantile Batten disease — read more about their story— caused by mutation(s) in the CLN6 gene. This particular variant is extremely rare and the disease course can differ significantly among cases, making it difficult to predict.
WHAT ARE THE SYMPTOMS AND WHEN DO THEY BECOME APPARENT?
Early symptoms of Batten disease can include progressive vision loss, seizures, behavioral changes, and loss of motor skills in previously healthy children. Symptoms get progressively worse, resulting in blindness, Parkinson-like symptoms, and dementia.
The different variants of the disease are distinguishable from one another in part by the age at which symptoms appear. Symptoms can appear as early as 6 months and as late as 43 years old. Children affected by Late Infantile Batten disease, including Charlotte and Gwenyth, typically begin to show symptoms between ages 2 to 4.[3]
HOW IS IT INHERITED AND HOW IS IT DIAGNOSED?
Batten disease is inherited as an autosomal recessive trait, meaning the same abnormal gene for the same trait is inherited from each parent. Both parents must be carriers and pass on the defective gene. The risk for two carrier parents to both pass the defective gene is 25% with each pregnancy.[1]
Typically, diagnosing Batten disease requires a combination of testing including blood or urine tests, skin or tissue sampling, EEG, electrical study of the eyes, brain scan using CT or MRI, and enzyme activity measurement. The only definitive diagnosis for Batten disease is through DNA analysis.[4]
Due to its rarity, diagnosing Batten disease is typically a long, painful journey for families who receive many false diagnoses before the final Batten determination.
HOW RARE IS BATTEN DISEASE?
Batten disease and other forms of NCL are rare – occurring in an estimated 2 to 4 of every 100,000 live births in the United States.[2]
Late Infantile Batten disease, specifically CLN6 which Charlotte and Gwenyth have, is particularly rare. Before we began our work, it was estimated that less than 10 children were currently living with this variant of the disease. However, we have met many more than that, and the number may be closer to 25.
WHAT IS THE PROGNOSIS?
As Batten disease is currently untreatable outside of investigative clinical trials, those affected will eventually become bedridden, fully dependent on their families or caretaker, and face premature death. Depending on the variant, Batten patients’ life expectancies can range from as young as 6 to adulthood (in the case of adult onset Batten disease).
WHERE CAN I LEARN MORE?
You can learn more by checking out our recommended resources.
HOW CAN I HELP?
You can make a difference in the lives of children and families struggling with Batten disease. The Charlotte and Gwenyth Gray Foundation is working to find a cure, as well as aiding families in their journeys with Batten disease through our scholarship program. Help us fund the effort to find a cure by donating, or contact us about other ways of getting involved.
[1] “Batten Disease.” NORD (National Organization for Rare Disorders). http://rarediseases.org/rare-diseases/batten-disease/
[2] “Batten Disease Fact Sheet.” National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/batten/detail_batten.htm
[3] “Understanding Batten – Prognosis.” Beyond Batten Disease Foundation. http://beyondbatten.org/understanding-batten/prognosis/
[4] “Understanding Batten – Diagnosis/Symptoms.” Beyond Batten Disease Foundation. http://beyondbatten.org/understanding-batten/diagnosis-symptoms/
